Contents
Properties of Radiation
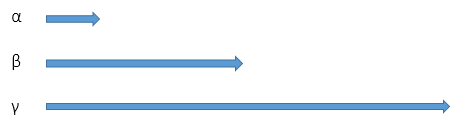
Range
Alpha particles can travel a few centimeters in air.
Beta particles can travel a few meters.
Gamma rays have an almost infinite range.
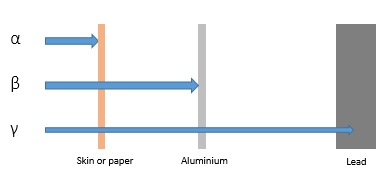
Penetration
Alpha particles can be absorbed by paper or skin.
Beta particles can be absorbed by thin aluminum.
Gamma rays can be absorbed by thick lead.
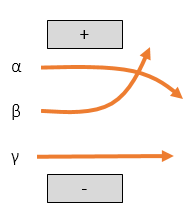
Magnetic and Electric Fields
Gamma rays will pass through a field unaffected as they have no charge and no mass.
Beta particles will be deflected towards the positive area of the field. They have a low mass, so they will be strongly deflected.
Alpha particles will be deflected towards the negative area of the field. They have a high mass, so they will be weakly deflected.
Danger
Which type of radiation is the most dangerous depends on the situation. Alpha particles are the most ionizing but are least likely to reach you due to their short range and low penetration. Gamma rays are the least ionizing but are the most likely to reach you due to their long range and high penetration.
You can be exposed to radiation in two ways:
- Irradiation: Exposure to a radioactive source outside of your body
- Contamination: Exposure to a radioactive source inside your body
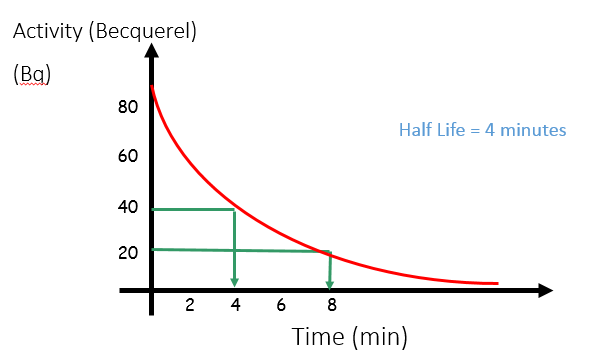
Half Life
The half-life is the time it takes for the (radio)activity of a substance to decrease by half.
Half-life can be calculated from a graph of the activity level of a radioactive substance over time. At any point on the graph (but it’s usually easiest to do it from the start), work out the time taken for the activity to drop by half – this is the half-life.
If the half-life is 4 minutes, then every 4 minutes, the activity will decrease by half.
Half-life can also be calculated numerically.
For example:
A sample of Bismuth-214 has an activity of 64 Bq. It has a half-life of 20 minutes.
- After 20 minutes (1 half-life), the activity will be 32 Bq (half the original activity).
- After 40 minutes (2 half-lives), the activity will be 16 Bq (half again).
- After 60 minutes (3 half-lives), the activity will be 8 Bq.
And so on.
Ionisation
Ionizing radiation has enough energy to move electrons from atoms. This is harmful to cells in your body and can cause cancer. Ionizing radiation can also be used to kill cancerous cells.
Alpha particles are the most ionizing.
Gamma rays are the least ionizing.
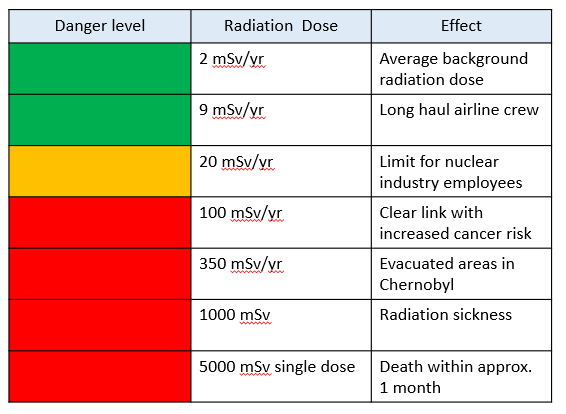
Background Radiation
Some elements emit ionizing radiation all the time. These radioactive elements are naturally found in the environment. Background radiation includes natural and man-made radioactive sources.
Exposure to radiation can happen in two ways:
- Irradiation - Exposure to radiation from a source outside your body
- Contamination - Exposure to radiation from a source inside your body
Carbon Dating
Living things contain some radioactive carbon-14. When the organism dies, it stops taking in carbon-14. The carbon-14 decays to carbon-12. The half-life of carbon-14 is 5600 years. The level of radiation emitted from the carbon-14 can be used to work out how long ago the organism died.
Worked Example:
A living bone emits 170 counts per minute. A bone from an archaeological site emits 50 cpm. The background radiation in the area is 10 cpm. How old is the bone? (Half-life of Carbon = 5600 years)
- Fresh bone – background radiation = 170 – 10 = 160 cpm
- Ancient bone – background radiation = 50 – 10 = 40 cpm
- Fresh = 160 cpm. 1 half-life later = 80 cpm. After 2 half-lives = 40 cpm
- The ancient bone is 2 half-lives old.
- Half-life = 5600 years
Ancient bone = 120,000 years old
Safety Precautions
The risk to the general public from radioactive materials is low, but people working with radioactive substances need to take precautions.
They can avoid contamination by:
- Careful storage of radioactive materials
- Wearing gloves/protective clothing
They can reduce irradiation by:
- Working behind lead shields/barriers
- Reducing time in at-risk areas
Monitor exposure using a film badge.
Using Radiation
When using radioactive materials, the properties of the source need to be matched to the specific need of that task considering:
- Range
- Penetration
- Ionisation
- Half-life
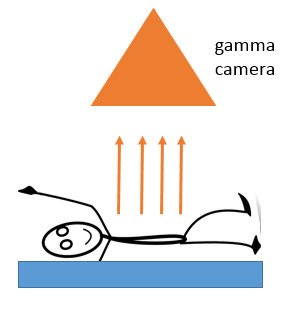
Medical Diagnosis - Gamma Camera
To use a scan from a gamma camera, the patient swallows a gamma tracer or has it injected into the bloodstream. The camera detects the gamma radiation emitted from the body, producing an image of the body parts containing the tracer.
A gamma source is used as it is the least ionizing and will pass through the body easily to be detected by the camera. It is important to choose a half-life that is long enough to allow the scan to be carried out but not so long that the patient remains contaminated for a long time.
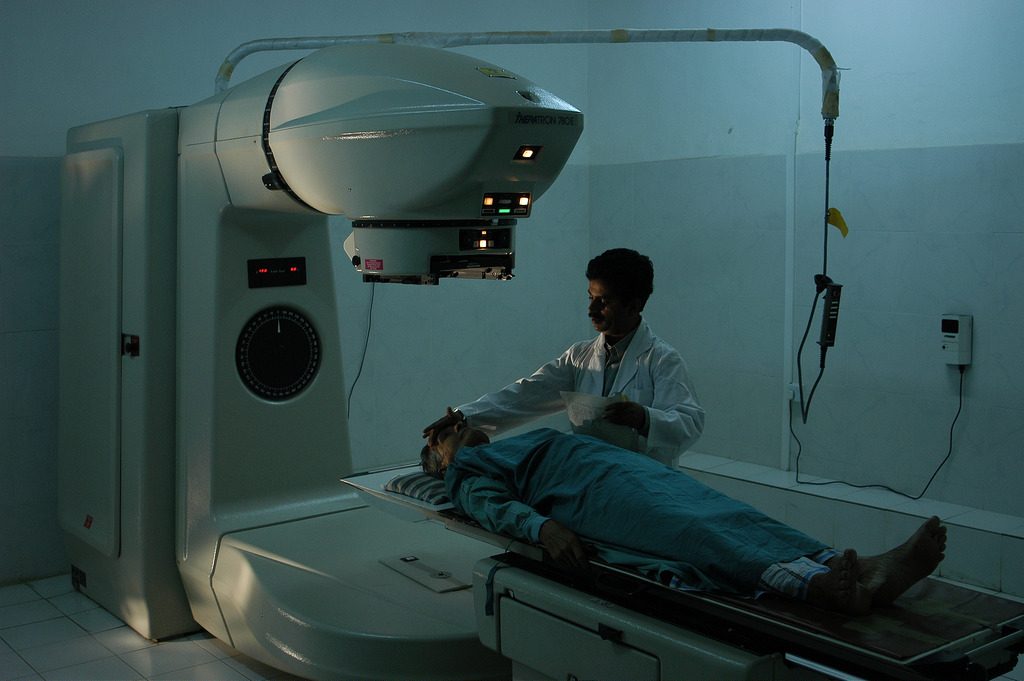
Medical Treatment – Radiotherapy
As ionizing radiation is dangerous to cells, it can be used to kill cancerous cells. The radiation is targeted at the tumor, but some damage to healthy cells is inevitable. Gamma or beta sources are usually used as alpha particles cannot penetrate the skin.
Sterilization
Radiation can be used to kill bacteria and therefore sterilize surgical equipment. The items can be sealed in airtight packaging before being irradiated, which means there is no chance of re-contamination before they are used.
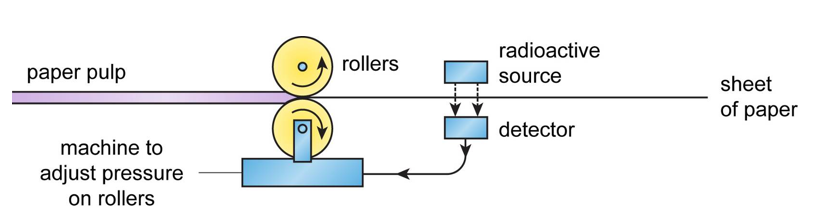
Measuring Thickness
Radioactive sources can be used to measure the thickness of materials that are being rolled out, such as paper or sheet metal. The source is positioned on one side of the material with a detector on the other side. A change in thickness will affect the amount of radiation passing through the material and being received by the detector. This can be used to change the pressure of the rollers to increase/decrease the thickness as necessary.
Beta particles are used for paper (as alpha particles cannot pass through paper and gamma rays would pass through too easily). Gamma rays are used for metal as alpha and beta particles cannot pass through.
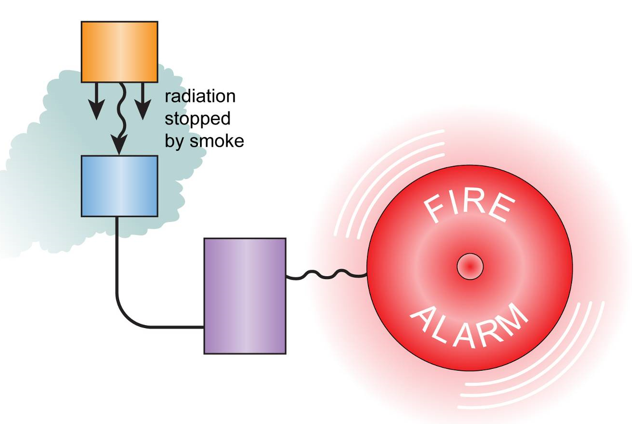
Smoke Detectors
Smoke detectors use an alpha source, which ionizes the air in a small gap in a circuit, allowing current to flow.
If smoke is present, it absorbs the alpha radiation, meaning current can no longer flow across the gap, triggering the alarm.
An alpha source is used because it cannot penetrate through smoke. Beta and gamma rays would not be suitable as they can pass through smoke.
- Which type of radiation is the most ionizing?
- Alpha
- Which type of radiation has no electrical charge?
- Gamma
- Which type of radiation has the largest range?
- Gamma
- The activity of a radioactive sample is 1440 Bq. 5 hours later it is 45 Bq. What is the half-life of this sample?
- Your answer should include: 1 hour / 1
- The half-life of Strontium-90 is 29 years. If you start with 1000 nuclei of Strontium-90, how many would you expect to be left after 87 years?
- 125