Contents
Protein Structure
Protein Structure
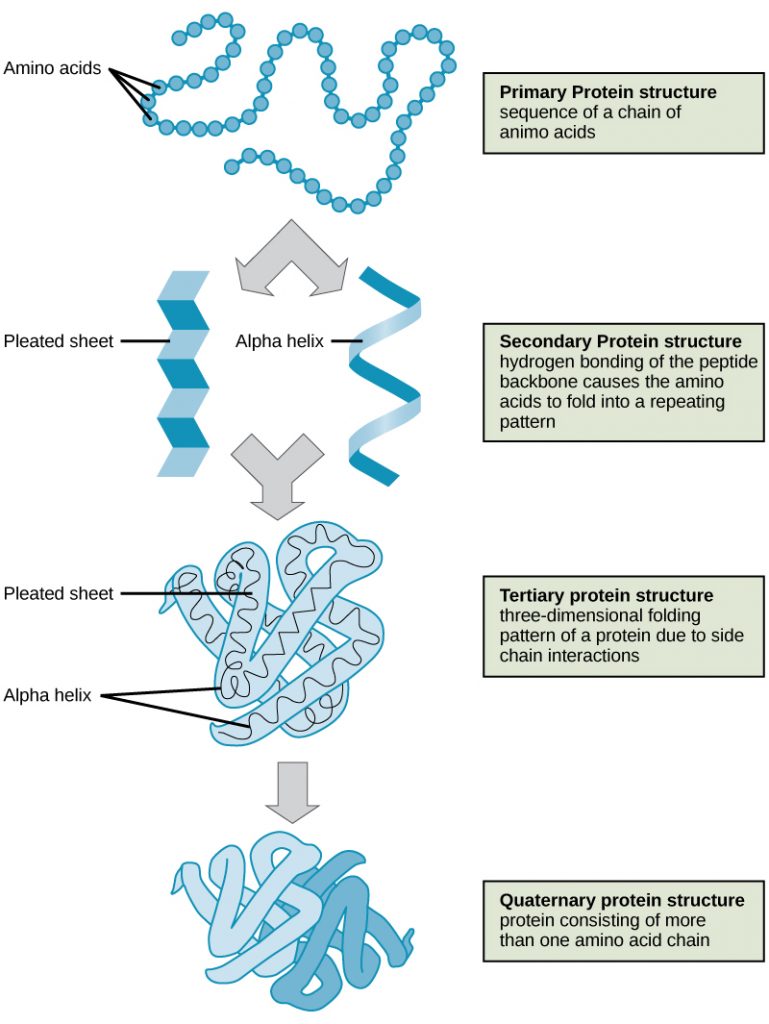
Proteins are large polymers made up of amino acids. These amino acids are arranged in a series of structures to create the finished 3D protein. There are up to four levels of structural arrangements in a protein, as shown in the image below, which will each be explained fully.
Proteins are polymer chains, or polypeptides, created on the ribosome in cells and are then further folded and modified in the Golgi apparatus.
Primary Structure
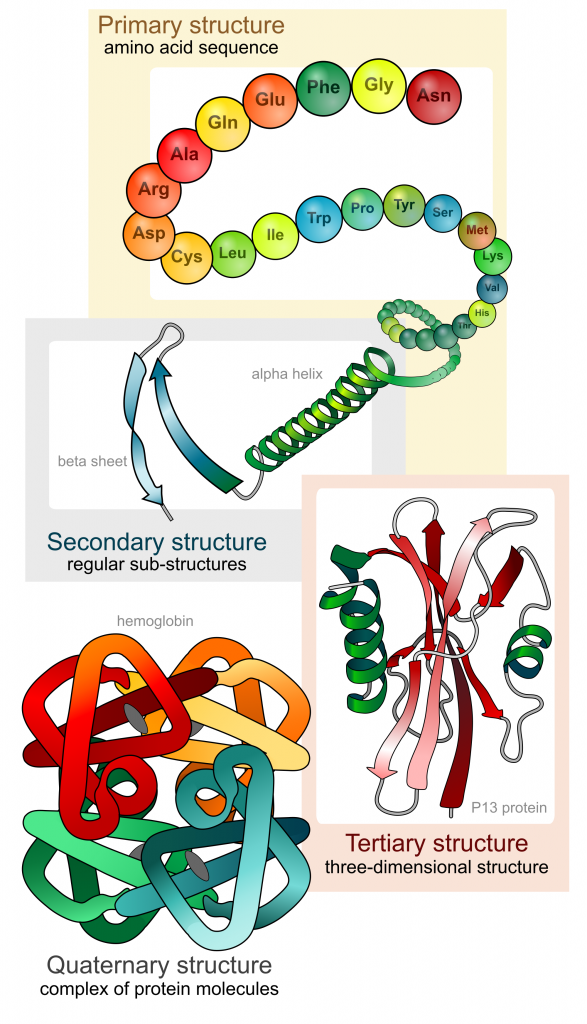
The first structure that forms in the creation of a protein is the polypeptide chain. Proteins are all made up of one or more polypeptide chains folded into highly specific 3D shapes. The exact definition of primary structure is: The sequence of amino acids in a polypeptide chain.
This would be a one-mark question, and it is essential to state the word ‘sequence’. The order in which the amino acids are bonded is determined by DNA. This specific order of amino acids will alter how the protein further folds and is bonded, enabling unique functions.
There are 20 different amino acids that can form the primary structure. The polypeptide chain is created by a series of condensation reactions occurring between amino acids.
- Stretch and challenge application question - What might cause a change to the amino acid sequence?
- Mutation
Secondary Structure
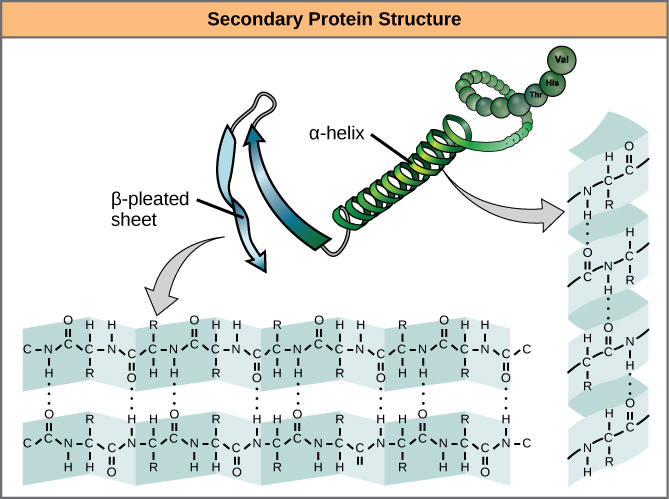
The sequence of amino acids causes parts of a protein molecule to bend into α helix shapes or fold into β pleated sheets. Hydrogen bonds between the carboxyl groups of one amino acid and the amino group of another are what hold the secondary structure in place. Hydrogen bonds are individually weak bonds, but when there are large numbers of them, they provide collective strength.
Describing the secondary structure would be a 2-mark question. The two marks would be for stating:
- Folding the primary structure into α helix shapes or fold into β pleated sheets
- Held in place by hydrogen bonds
Tertiary Structure
The secondary structure is bent and folded to form a precise 3D shape. This unique 3D shape is held in place by hydrogen bonds, ionic bonds, and sometimes disulfide bonds. Disulfide bonds (di meaning 2) only form between the R-groups of two amino acids that contain sulfur. Describing the tertiary structure of a protein is a 3-mark question. The 3 marks are typically:
- The further folding of the secondary structure
- To create a unique 3D structure
- Held in place by hydrogen, ionic, and disulfide bonds.
Make sure you always mention the bonds involved when describing protein structures; there are always marks for this because without these bonds, the unique shapes are not maintained.
Quaternary Structure
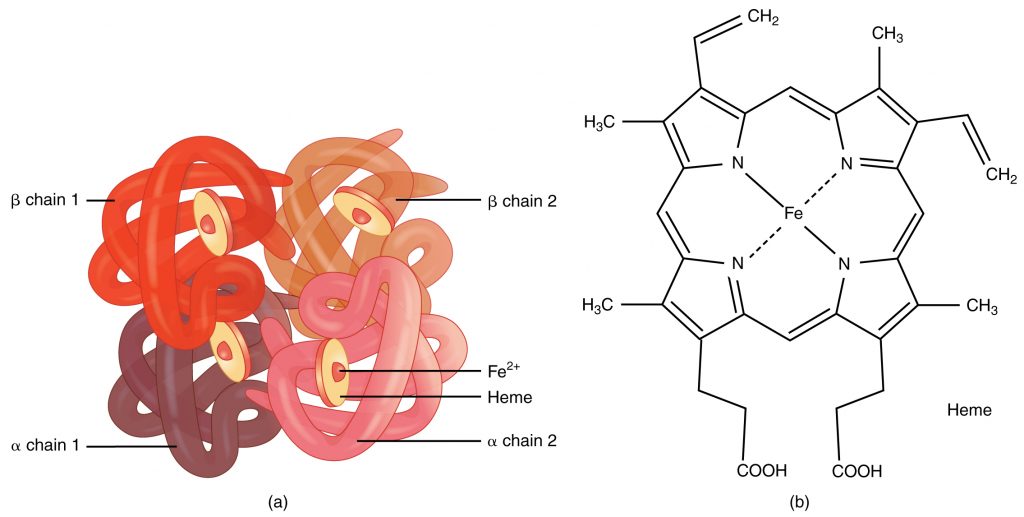
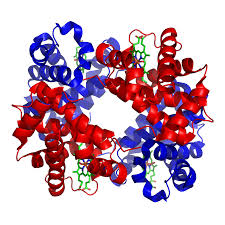
A protein that is made up of more than one polypeptide chain is a quaternary structure protein. It is still folded into a 3D shape and held by hydrogen, ionic, and disulfide bonds. Hemoglobin is the typical example given. Hemoglobin is made up of 4 polypeptide chains.
In the diagram of hemoglobin, you can also see extra molecules attached that are not part of the polypeptide chains. Any group that is attached to a protein but is not made up of amino acids is known as a prosthetic group. Iron is the prosthetic group in hemoglobin. A protein that has a prosthetic group can be described as a conjugated protein, which simply means a non-protein group is added onto it.
Fibrous & Globular Proteins
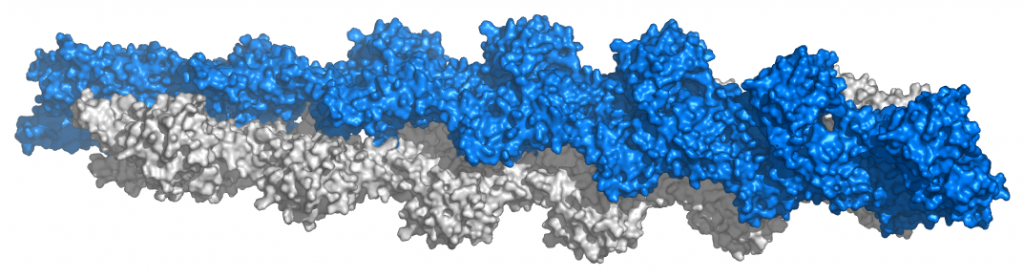
The 3D folding in tertiary and quaternary proteins generally results in either a shape that is spherical, called globular proteins, or a long rope-like shape, called fibrous proteins. Fibrous Protein Fact File:
- Polypeptide chains form long twisted strands linked together
- Stable structure
- Insoluble in water
- Strength gives structural function
- e.g., collagen in bone and keratin in hair.
Globular Protein Fact File: Polypeptide chains ‘roll up’ into a spherical shape
- Relatively unstable structure
- Soluble
- Metabolic functions
- e.g., all enzymes, antibodies, some hormones (e.g., insulin), hemoglobin.